Abstract
Introduction. Myeloproliferative neoplasms (MPNs) like Essential Thrombocythemia (ET) presents with higher platelet counts and driver mutations in JAK2, CALR or MPL. Thrombotic and hemorrhagic complications are main causes of morbidity/mortality. Platelet functional alterations have been previously reported in patients with ET, also higher reticulated platelet fraction and higher aggregation potential compared to healthy controls. The aim of this study was to investigate platelet activity and thrombin generation (TG) in patients with ET with different molecular mutations.
Methods.Thirty-eight adult patients with ET according to 2017 WHO classification and 31 healthy controls were included. Patients were not receiving cytoreductive treatment, and antiaggregant drugs were stopped 7 days prior to sample collection. Patients group included JAK2 mutations (n=18), CALR (n=7), MPL (n=2) and triple-negative (TN)(n=11). Platelet characteristics and functional assessment included flow cytometry and TG was tested using Calibrated Automated Thrombogram (CAT) with either PPP-low [1pM tissue factor (TF) and 4 µM phospholipids (PL)] or PRP (1pM TF) or MP (4 µM) reagents as triggers. As platelet volume in ET patients was different from controls, the number of fibrinogen (FG) receptors as well as the PAC binding per FG receptor per platelet was corrected using the platelet volume. Two MPL+ patients were excluded from the analysis due to the low number.
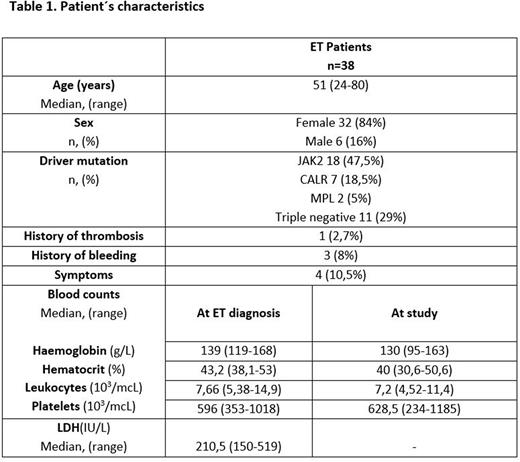
Results.ET patients included 32 females and 6 males with median age of 51 years (24-80), patients characteristics are shown in Table 1. Median platelet count was 628,5 x109/L (234-1185) and median platelet volume was 6,9fL (5,2-11).No differences in the haemoglobin (Hb), Hematocrit and lactate dehydrogenase (LDH) at ET diagnosis were found except for a significantly higher platelet count in CALR+ patients (p<0.05).
The number of FG receptor per platelet is lower in ET patients compared with controls (p<0.001).
Fibrinogen receptors on platelets from ET patients showed decreased capacity to be activated by agonists TRAP and ADP, but as ET platelets had lower FG receptors, when activation was corrected by number of FG receptors, no differences among groups were seen.
Platelets from JAK2+ and TN patients exposed P-selectin levels similarly to controls after agonist stimulation with TRAP, platelets from CALR+ patients exposed significant lower P-selectin levels respect to controls (p<0.01) and TN patients (p<0.01).
Decreased CD63 exposure was noted on CALR+ group after TRAP stimulation (p<0.01)compared to healthy controls and TN patients. Von Willebrand Factor (vWF) receptor expression (CD42a) was lower in ET patients regardless the molecular status (p<0.05).
Plasma from JAK2+ patients had less thrombogenic potential according to the reduced values of endogenous thrombin potential (ETP) (p<0.01) and peak (p<0.05) compared to controls and lower ETP compared to TN patients (p<0.05). TG capacity in CALR+ showed a decrease in ET compared to controls (p<0.01), TN patients and JAK2+ (p<0.05). In PL-dependent TG, a significantly diminished peak was observed in CALR+ compared to controls, TN and JAK2+. TG depending on TF on microparticles was similar to controls in JAK+, TN and CALR+ patients.
Conclusions.Our results suggest that molecular profile in ET patients may influence TG. A significant decrease in the thrombogenic potential was found in JAK2+ and CALR+ patients. Regarding platelet function, some alterations were found in CALR+ group.
Collaborative studies increasing number of patients included should be performed to identify a characteristic pattern of haemostasis for each mutation that could help to adjust thrombotic prophylaxis in ET patients.
Disclosures
Gasior Kabat:Brystol Myers Squibb: Other: Advisory Board ; Eusa Pharma: Speakers Bureau; Novartis: Other: Advisory Board , Speakers Bureau. Alvarez Román:Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; CSL-Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Octapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Biomarin: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other; Grifols: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia. Hermans:oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Research Funding; oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Honoraria; oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, BioMarin, Octapharma, CSL Behring: Consultancy. Jiménez Yuste:Pfizer: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BioMarin: Consultancy; Sobi: Consultancy, Honoraria, Research Funding; NovoNordisk: Consultancy, Honoraria, Research Funding; Octapharma: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding. Butta:Roche: Speakers Bureau; Novo-Nordisk: Speakers Bureau; Takeda: Research Funding, Speakers Bureau; CSL-Bering: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.